· Term Carbohydrate is derived from French term hydrate
de Carbone
means
Hydrate of Carbon.
·
Basically carbohydrates are Polyhydroxy Aldoses or Ketoses
and their condensation products.
o
Aldoses – (-CHO) group at one end. E.g.- Glucose,
Glactose
o
Ketoses – (=C=O) group in the chain. E.g. – Fructose,
Ribulose
·
Many small carbohydrates are sweet in test so they are
called Sugar or Saccharide.
· Simplest carbohydrate
·
Cannot be hydrolyzed into smaller carbohydrate
·
General Formula CnH2nOn
On the basis of number of carbon atoms monosaccharides can be classified into following groups :-
·
Structure of some Important
Monosaccharides
Glyceraldehyde
& Dihydroxyacetone
Ribose
& Deoxyribose

Glucose
·
Blood sugar
·
Grape sugar
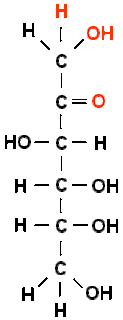
Fructose
·
Sweetest of all natural sugars
·
Fruit sugar except Grape, Found in honey as levulose
Ring forms
·
Pyranose – 6 membered ring containing 5C and 1O
atoms
·
Furanose – 5 membered ring containing 4C and 1O
atoms
α and β forms
· α form – (-OH ) group of C atom nearer to oxygen is downward
· β form – (-OH) group of C atom nearer to oxygen is upward
D and L form
·
D form – Dextro(right) rotation of light after
passing through the carbohydrate solution
· L form – Laevo (left) rotation of light after
passing through the carbohydrate solution
§ D form is more
common in living world
Derived Monosaccharide
Those compounds which are formed after addition or deletion of atom or
group of atoms are subjected in this group.
On the basis of added or deleted entity derived monosaccharides are
placed in following groups:-
·
Deoxy sugar – Formed after
deletion of one oxygen from any monosaccharide. E.g. – Deoxyribose sugar
·
Sugar Acid – Formed after oxidation of the monosaccharide. E.g. –
Glucuronic Acid
Amino Sugar – (-NH2) group get attached with the monosaccharide.
E.g. - Glucosamine
Sugar Alcohol – Formed after
addition of (-OH) group with monosaccharide. E.g.- Sorbitol
To download this tutorial in pdf click here










No comments:
Post a Comment